Our Excited-State PCET at High Pressure Featured in Nature News & Views!
2025-05-27
Our recent study on excited-state proton-coupled electron transfer (PCET) under high pressure has been featured in Nature News & Views in an article 'Squeezing the mechanism out of photochemical reactions'. The highlight emphasizes our mechanistic insights into how pressure influences PCET pathways. We’re pleased to see our work recognized in this context!
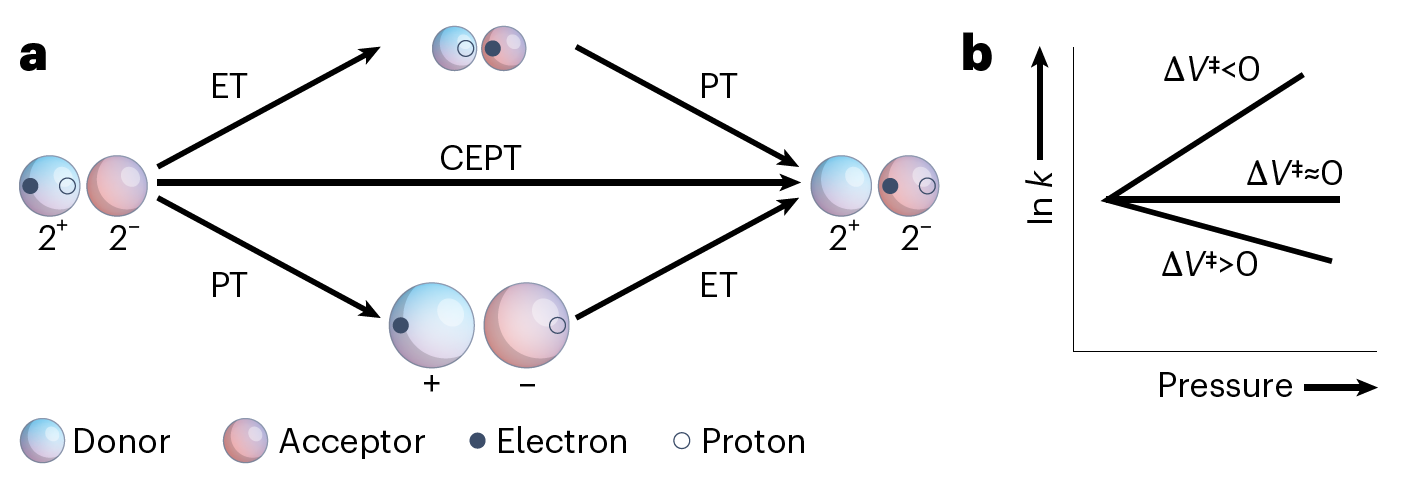
a, Square scheme showing different mechanistic pathways for a PCET reaction. Blue and red spheres represent the electron and proton donor and acceptor states, respectively, and include the surrounding solvent. Increased charge on ionic solutes intensifies electrostriction and reduces the partial molar volume (indicated by decreasing sphere size).
b, The charge-transfer rate constant k as a function of pressure under different activation volume regimes. The negative activation volume associated with electron transfer (ET) will yield faster rates at higher pressures. Conversely, the positive activation volume associated with proton transfer (PT) will yield slower rates at higher pressures. Because the charge of the donor and acceptor states remains the same in the concerted electron proton transfer (CEPT) reaction, minimal changes in electrostriction are expected. As a result, the activation volume approaches zero and rates have diminished sensitivity to pressure differences. ΔV‡ is the activation volume.
Rosichini, A.; Glover, S.D. Squeezing the mechanism out of photochemical reactions. Nature Chemistry. (2025). DOI: 10.1038/s41557-025-01823-x